Home
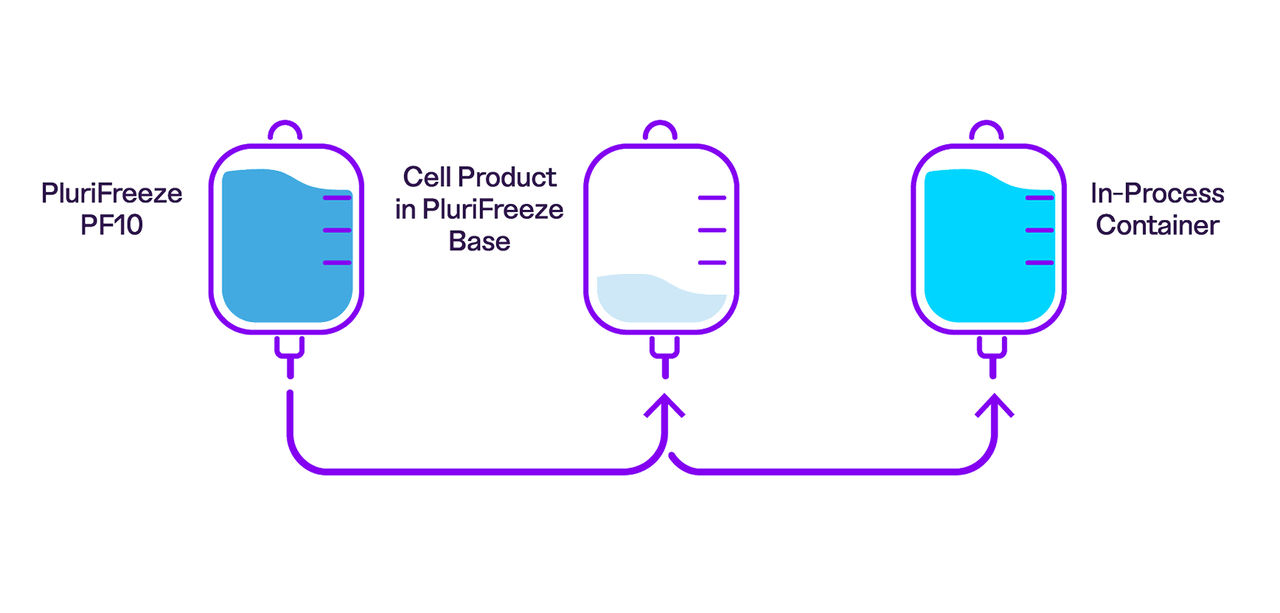
Learn how to use the PluriFreeze™ Cryopreservation System in a closed workflow
We've updated the protocol for the PluriFreeze Cryopreservation System to provide more detailed instructions for a wider variety of use cases, including large-scale cryopreservation in a closed system, which is facilitated by PF10's low viscosity.
Looking for 0.9% Saline Solution? We've got it in stock.
For your convenience, our off-the-shelf solutions include both RUO and GMP-grade options in sizes ranging from 10 mL tubes to 1000 mL bottles to 15 L carboys. Custom formulations and formats are also available by request.
Have you read our new white paper?
Streamlining the Transition from RUO to GMP:
Key Process Development Considerations to Accelerate Scale-Up and Tech Transfer for Emerging Therapies

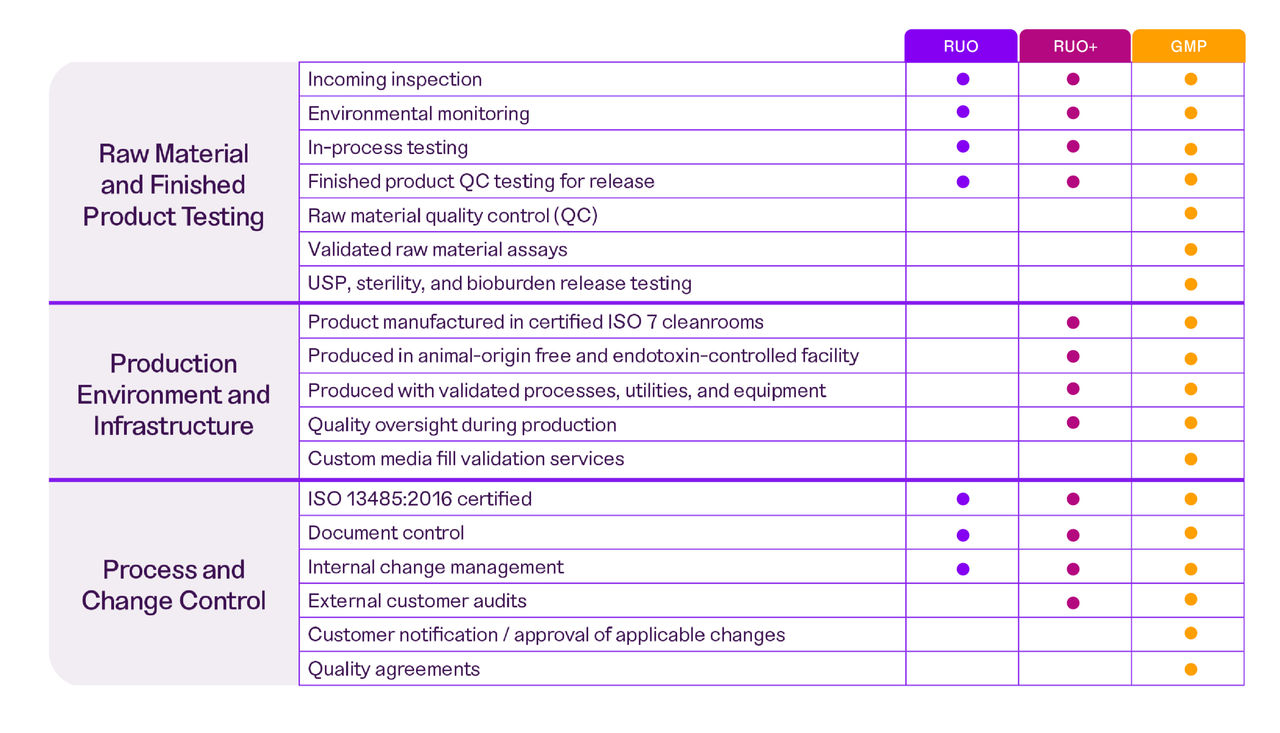
Get to know our quality control testing
We follow rigorous testing and documentation procedures that go beyond industry standards to ensure you get reproducible results. Our industry-trained experts can also help design a custom testing program to meet your specific requirements.

Streamline your scale-up with single-use bags
Did you know that our RUO and GMP buffers, broths, and water are available in bag sizes ranging from 1 L to 200 L? This is just one of the flexible format options we offer to help support your tech transfer and clinical manufacturing needs.

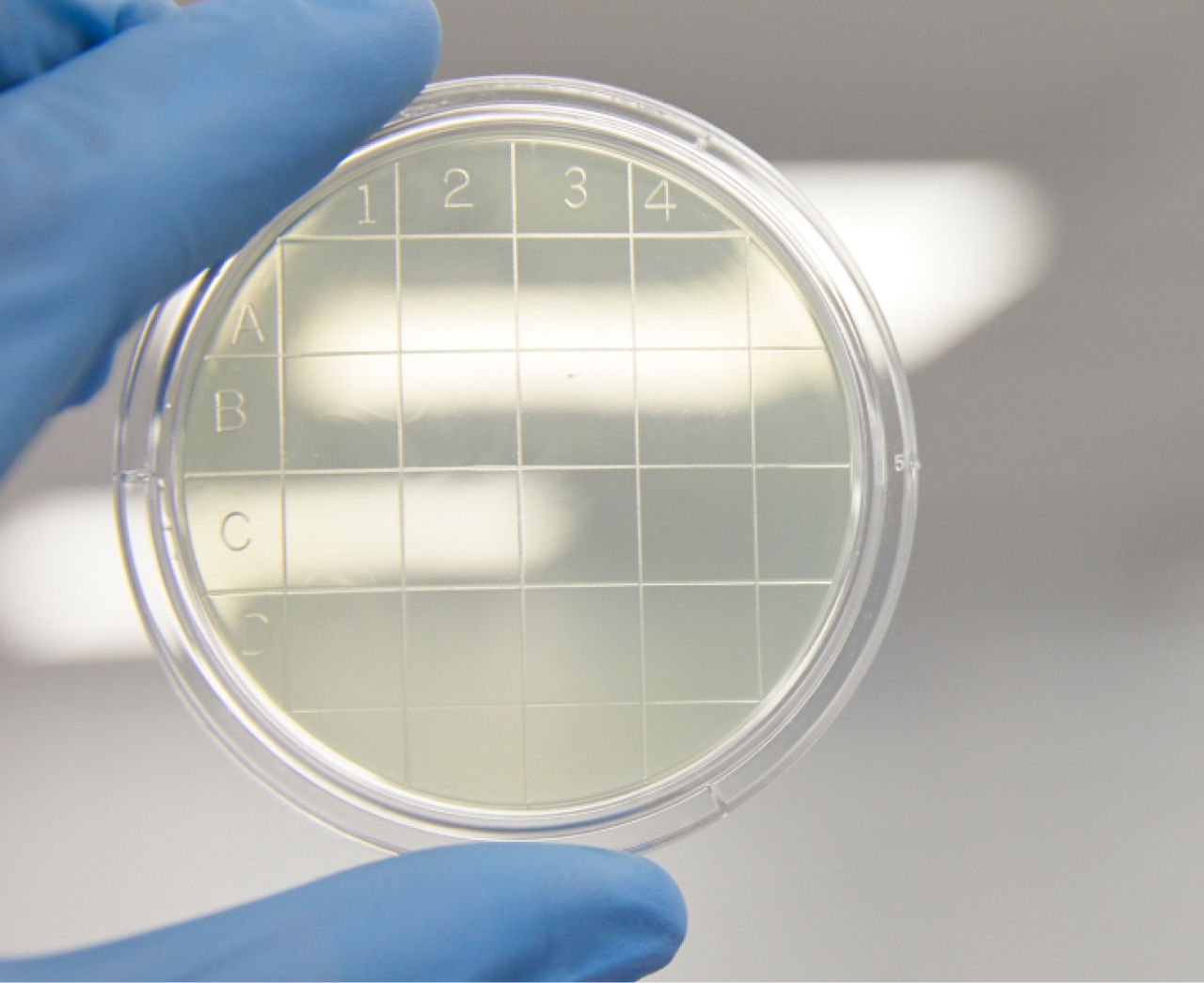
New product: Environmental monitoring settle plates
You can now find Tryptic Soy Agar Settle Plates in our online catalog. Designed to be your first line of detection of microbial contamination, these are the same high-quality plates we use to perform environmental monitoring in our own cleanrooms.
Discover our new custom services
We are now offering a RUO+ manufacturing grade to streamline your transition from RUO to GMP, as well as Express•Tek℠ Production services to get your custom formulation into production in days instead of weeks.
- Pluristyx
- Saline
- White Paper
- QC testing
- Bags
- Environmental monitoring
- Custom services
- Culture
- Cryopreservation
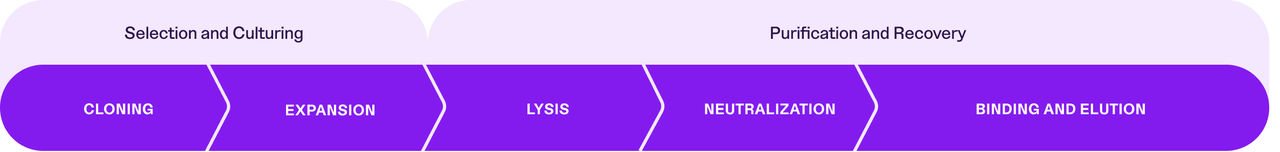
- Extraction and Purification
- Analytics
- Environmental Monitoring
A trusted partner that
can help you scale
With 30 years of experience manufacturing custom reagents, we have finessed the rapid turnaround of customer-specified products. Our new state-of-the-art facility has increased our footprint to over 180,000 square feet, enabling us to better serve our customers by providing the scale and flexibility needed for GMP manufacturing.
Find out what’s possible
Talk to our consultants today to find the ideal off-the-shelf or custom solution that can help you achieve your goals.
Our team is here to help
Monday to Friday
8:00AM–5:00PM PT
- AAV Workflow
- Plasmid Workflow
Find out what’s possible
Talk to our consultants today to find the ideal off-the-shelf or custom solution that can help you achieve your goals.
Our team is here to help
Monday to Friday
8:00AM–5:00PM PT
Where to find us
For your convenience, we work with a global network of distributors.
You'll always find the widest selection of our products right here, but you can also order our off-the-shelf solutions from our distributors.
- AAV Workflow
- Plasmid Workflow
Bridge the gap from RUO to GMP with
our range of manufacturing grades
Find out what’s possible
Talk to our consultants today to find the ideal off-the-shelf or custom solution that can help you achieve your goals.
Our team is here to help
Monday to Friday
8:00AM–5:00PM PT
We'll come to you
Want to learn more about what we can do for your company?
Schedule a time for our team to come give a tailored presentation about how our products and services can address your specific needs.
- Acids and Bases
- Additives
- Alcohols
- Culture Media
- Buffers
- Detergents and Surfactants
- Salts
- Sugars
- AAV Workflow
- Plasmid Workflow
Find out what’s possible
Talk to our consultants today to find the ideal off-the-shelf or custom solution that can help you achieve your goals.
Our team is here to help
Monday to Friday
8:00AM–5:00PM PT
Take a tour of our facilities
Come see our state-of-the-art facilities in person.
Schedule a tour to get an in-depth understanding of our manufacturing capabilities. Our QMS and production experts can answer your detailed questions and discuss how we can meet the needs of your specific application.