PROTOCOL
How to use the
PluriFreeze™ Cryopreservation System
To help you get successful outcomes, our team of cell therapy and reagents experts has put together a proven protocol, and we are here to help with any questions you may have about how to optimize your specific workflow.
Introduction
How the PluriFreeze Base and PF10 work together
The PluriFreeze Cryopreservation System consists of two products designed to work together to optimize your cryopreservation outcomes: PluriFreeze Base, a unique protective wash that mimics intracellular space and provides metabolic support across your workflow, and PluriFreeze PF10, a low-viscosity freezing medium with 10% DMSO that simplifies scale-up and process automation.
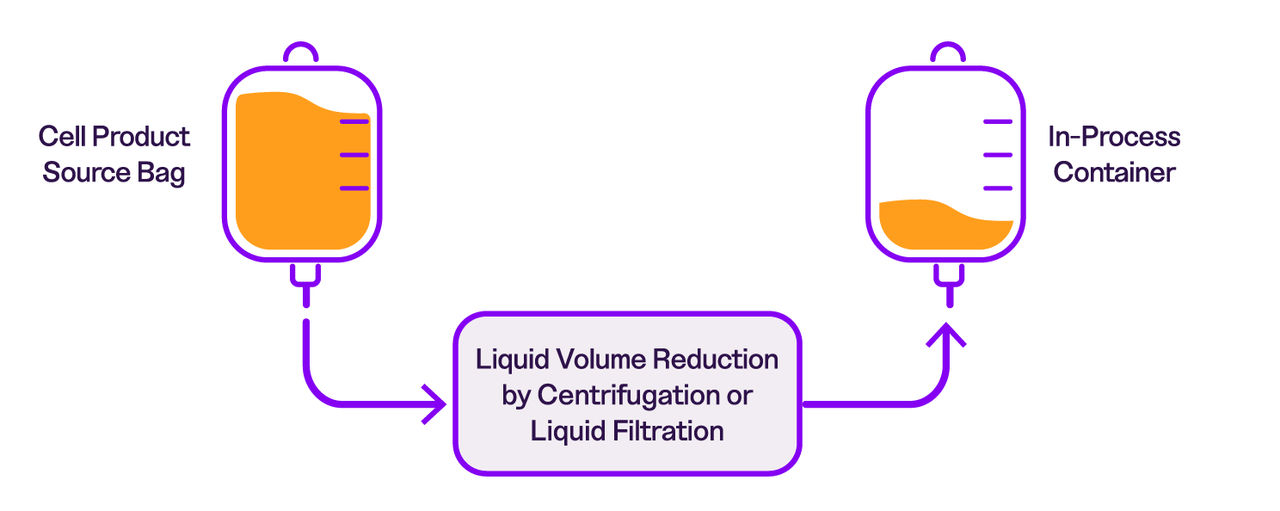
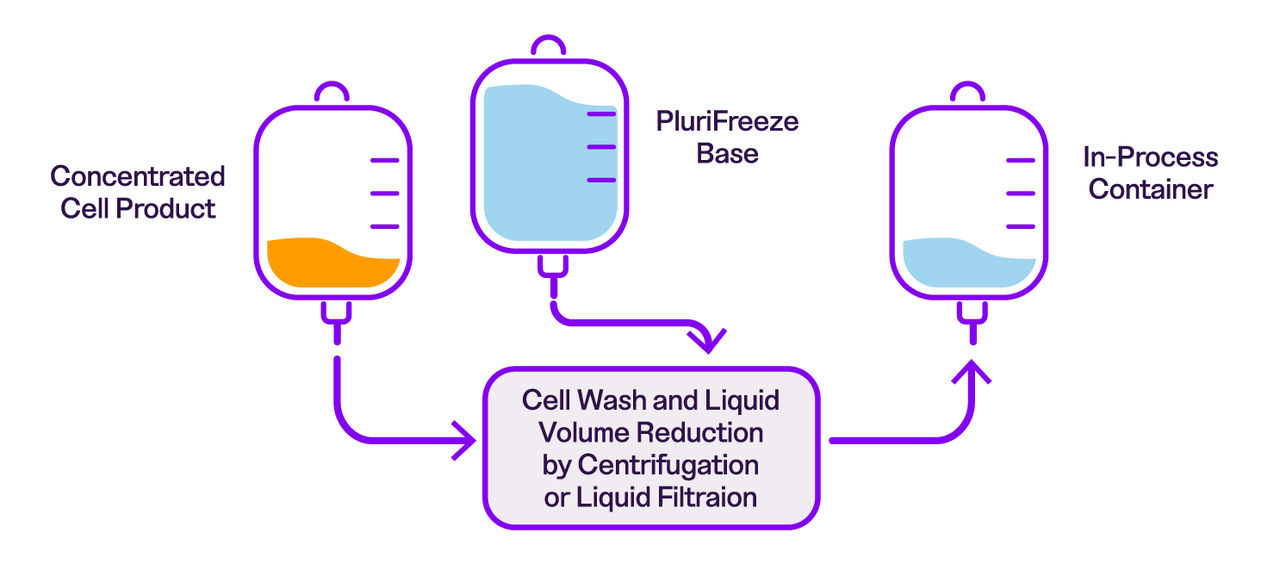
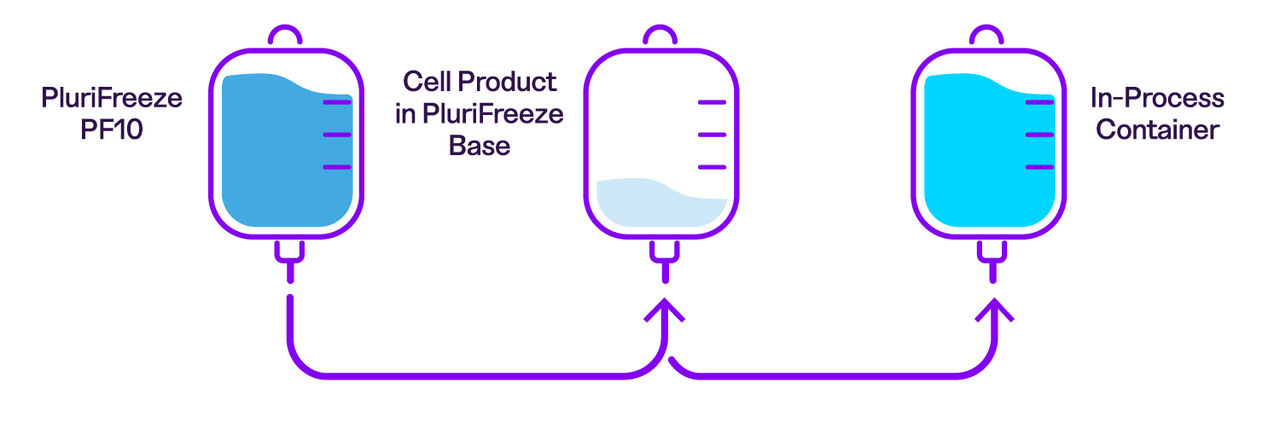
While we recommend using both products together, PluriFreeze Base can also be used independently during cold-hold steps in your workflow. The figure below illustrates where PluriFreeze Base and PluriFreeze PF10 fit within a typical cell therapy manufacturing process.

Benefits of adding the PluriFreeze Base into your cryopreservation workflow:
• Acts as a hyperosmotic holding solution for cell products: This is useful for large-scale manufacturing processes where processing time exceeds the recommended DMSO exposure time or when maintaining cell products without freezing.
• Easily dilute PluriFreeze PF10 cryomedia to achieve a specific DMSO concentration: Simply mix PluriFreeze PF10 with PluriFreeze Base at the appropriate ratio to obtain the desired final DMSO concentration.
• Use as a wash medium prior to formulating cells in PluriFreeze PF10 freezing medium: With a complementary formulation to PluriFreeze PF10, PluriFreeze Base acts as a protective wash by mimicking intracellular space and providing additional metabolic support.
Note: PluriFreeze Base is not intended to be used as a cell culture medium and should not replace growth medium for quenching steps during dissociation or for maintenance of cells.
- Closed-System Workflow
- Small-Scale Applications
- Cold Holds and Transport
- Tips for Success
The low viscosity of the PluriFreeze system enables efficient fluid handling and improved washout of residual media components during closed-system, large-scale processing.
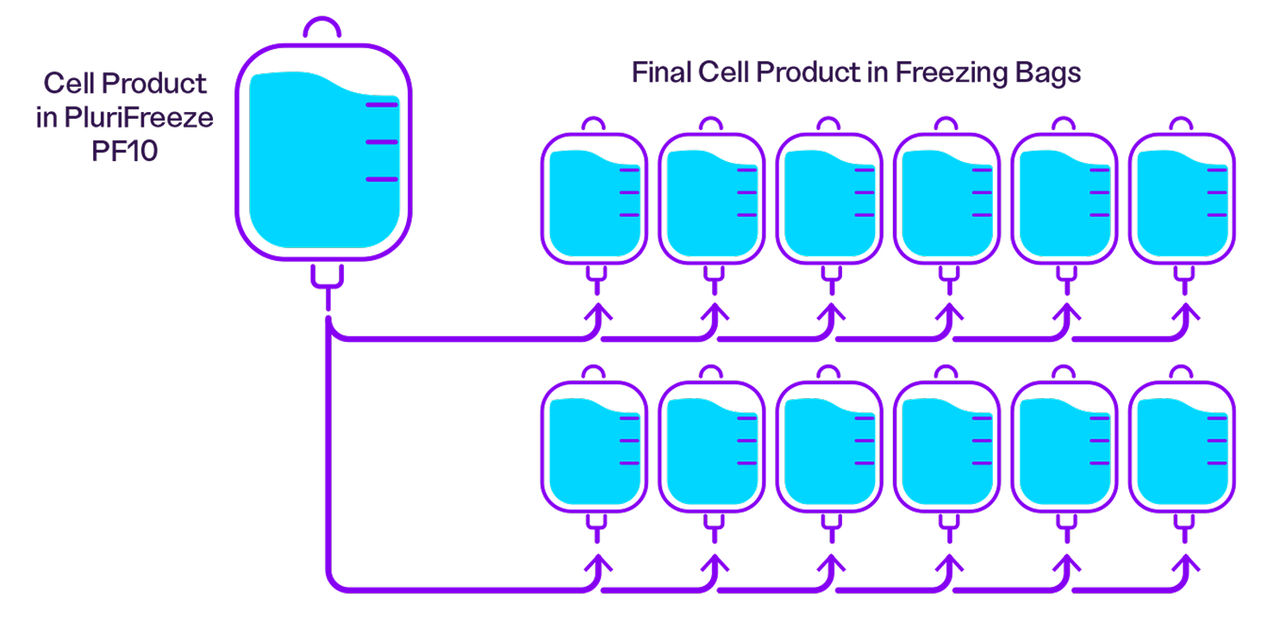
In small batch processing, PluriFreeze Base can help minimize DMSO and centrifugation stress while allowing for accurate cell counting prior to cryopreservation in PluriFreeze PF10. The figure below shows the process workflow for small-scale cryopreservation applications.

Prior to beginning, ensure that all supplies used for cryopreservation are chilled to 2-8°C.
• If using a controlled-rate freezer, ensure there is a sufficient supply of liquid nitrogen (LN₂) and fill the LN₂ vapor-phase carrier to equilibrate to a temperature ≤ 150°C. Follow the manufacturer's instructions for operation of the controlled-rate freezer and carrier. Place a pre-labeled storage box for cryovials into the carrier for temperature equilibration.
• If using an isopropanol freezing container, fill the outer chamber with 100% isopropanol to the fill line and chill at 2-8°C for at least one hour before use.
• Aliquot the appropriate amount of cryomedia (+10% overage) and chill at 2-8°C for at least one hour before cell processing.
•Pre-label and chill cryovials before starting the cryopreservation procedure.
For cells, tissues, or organ products that don't cryopreserve well and must be transported as live cultures, PluriFreeze Base serves as a hyperosmotic holding solution that can temporarily replace growth medium. Additionally, PluriFreeze Base may be used to hold cells in a quiescent state for in-process analytical testing, particularly in large-scale manufacturing processes. We recommend holding tissues/cells in PluriFreeze Base no more than 72 hours at 2-8°C. Optimal hold time and other parameters should be determined and validated for each particular tissue/cell type.
Products used in this protocol